The generic version of Renagel, known as sevelamer hydrochloride, is expected to become available in the near future, specifically projected for release in early 2024. This development will provide patients with more affordable options for managing elevated phosphate levels, particularly those undergoing dialysis.
Pharmaceutical companies are actively preparing for the launch, with several manufacturers poised to enter the market. This increased competition is likely to lead to better pricing and wider accessibility for those in need of treatment.
Healthcare providers should stay informed about the upcoming availability of generics to guide patients effectively. By discussing treatment options and their implications, doctors can ensure patients receive the best possible care tailored to their financial circumstances.
- Renagel Generic Release Date
- Availability and Benefits
- Usage Recommendations
- Understanding Renagel and Its Uses
- Indications for Use
- Dosage and Administration
- What Is a Generic Medication?
- Overview of the Renagel Patent Status
- Current Patent Details
- Implications for Generic Availability
- Expected Timeline for Renagel Generic Release
- Current Market Availability of Renagel
- Potential Manufacturers of Renagel Generics
- Regulatory Processes for Generic Approval of Renagel
- Submission and Review
- Market Entry and Post-Market Surveillance
- Implications of Generic Availability on Pricing
- Comparative Efficacy of Renagel and Its Generics
- Clinical Evidence
- Patient Experience
Renagel Generic Release Date
The generic version of Renagel, known as sevelamer hydrochloride, has been available since 2014. This medication is utilized primarily for managing hyperphosphatemia in patients with chronic kidney disease who are on dialysis. The introduction of the generic version has significantly improved access to treatment for many patients.
Availability and Benefits
Pharmacies are widely stocking sevelamer hydrochloride, making it a practical choice for individuals seeking affordable alternatives. Since its release, this generic formulation has maintained the same efficacy as its brand-name counterpart, allowing patients to manage their phosphorus levels effectively.
Usage Recommendations
Consult healthcare providers regarding dosage and administration. Adjustments may be necessary based on individual health conditions. Regular monitoring of phosphorus levels while on sevelamer hydrochloride is advisable to ensure optimal results and prevent potential complications.
Understanding Renagel and Its Uses
Renagel is a phosphate binder specifically designed for patients with chronic kidney disease (CKD) undergoing dialysis. This medication aids in managing elevated serum phosphorus levels by binding dietary phosphorus, reducing its absorption in the gastrointestinal tract. Effectively controlling phosphorus levels is crucial for mitigating complications such as cardiovascular disease and bone disorders in individuals with kidney impairment.
Indications for Use
Renagel is primarily indicated for patients who show elevated serum phosphorus levels due to advanced kidney disease. Regular monitoring of phosphorus levels is advisable to tailor dosage effectively. Additionally, Renagel is often used in conjunction with dietary modifications to enhance its therapeutic benefits.
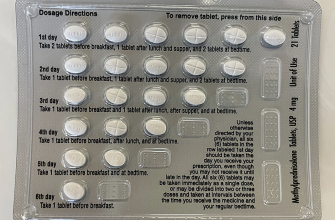
Dosage and Administration
The dosage of Renagel varies depending on individual patient needs and serum phosphorus levels. Typically, it’s taken with meals to maximize its binding effect on phosphorus. Healthcare providers usually recommend starting with a lower dose, adjusting as required based on laboratory results. It’s essential to follow the prescribing physician’s recommendations to optimize treatment outcomes.
| Condition | Recommended Action |
|---|---|
| Elevated Phosphorus | Administer Renagel with meals |
| Serum level monitoring | Adjust dosage based on levels |
| Dietary phosphorus intake | Implement dietary restrictions |
Addressing phosphorus levels proactively can significantly impact a patient’s quality of life. Consulting with a healthcare provider about Renagel’s role in a comprehensive treatment plan is advisable.
What Is a Generic Medication?
A generic medication is a drug that contains the same active ingredients as the brand-name counterpart and is used to treat the same conditions. Generics are developed after the patent on the brand-name drug expires, allowing other manufacturers to produce the medication.
Generic medications are required to meet the same standards of quality, safety, and efficacy established by regulatory authorities like the FDA. This means they must perform in the same way as their branded versions. Patients can expect similar results when using generics, though they may differ in inactive ingredients, such as fillers or colorings.
Often, generics are available at a lower cost than brand-name drugs, making them an attractive option for those seeking affordable medication. Patients should consult their healthcare provider before switching to a generic to ensure it’s suitable for their treatment plan.
In summary, generic medications provide a high-quality alternative to brand-name drugs, helping individuals save money without sacrificing effectiveness. Always discuss any medication changes with your doctor to ensure you receive the best care.
Overview of the Renagel Patent Status
The Renagel patent protections significantly influence the availability of generic versions of the drug. As of now, the key patents related to Renagel (sevelamer hydrochloride) are set to expire in 2026. This expiration date opens the door for generic manufacturers to submit their applications to produce their versions of this medication.
Current Patent Details
- The primary patent for Renagel was granted in the early 2000s, covering its unique formulation and specific uses.
- Additional patents related to manufacturing processes and formulations may also exist, impacting the timeline for generic entry.
- Patent litigation may arise, potentially delaying the release of generics beyond the expiration date.
Implications for Generic Availability
- Generic drug manufacturers can file Abbreviated New Drug Applications (ANDAs) for Renagel once the patents expire.
- Anticipated competition from generics can lead to lower prices for consumers and increased access to treatment.
- Keep an eye on the developments regarding patent disputes, as they could influence the timeline for generic launch.
Understanding the patent status allows healthcare providers and patients to anticipate potential changes in the market and plan for cost-effective treatment options. Regularly check for updates from reputable sources for the most current information.
Expected Timeline for Renagel Generic Release
The release of a generic version of Renagel is anticipated to occur within the next few years. The FDA approval process is the primary factor influencing this timeline.
- Current Status: As of now, no generic versions have received FDA approval. Companies are actively pursuing this opportunity, which indicates strong interest in the market.
- Clinical Trials: Several manufacturers are conducting bioequivalence studies. Results from these trials are crucial for gaining FDA approval.
- Expected Approval: Analysts predict that generics may receive approval as early as 2025, depending on the progress of clinical trials and regulatory reviews.
Stakeholders, including pharmacists and patients, should stay informed about the developing situation. Monitoring announcements from pharmaceutical companies can provide updates on when generics will be available.
- Keep an eye on FDA publications for generic drug approvals.
- Subscribe to industry news outlets for the latest updates.
- Consult healthcare providers for recommendations once generics are available.
Staying proactive ensures that individuals can take advantage of generics, leading to cost savings and enhanced access to medication.
Current Market Availability of Renagel
Renagel, primarily used to manage phosphate levels in patients with chronic kidney disease, remains available on the market. Its primary formulation, sevelamer hydrochloride, is offered as tablets and powder for suspension. Various manufacturers have produced generic versions that ensure competitive pricing and accessibility.
The introduction of generics has increased options for patients and healthcare providers. These alternatives are particularly beneficial for those seeking cost-effective treatments without compromising quality. Market supply for both the brand name and generic forms is stable, with widespread distribution across pharmacies in the United States and select international markets.
Key players in the generic segment include major pharmaceutical companies, which have received approval from the FDA. Patients searching for Renagel or equivalents should check with local pharmacies for availability, as stock levels can vary.
Here’s a summary of the current market availability:
| Formulation | Available Manufacturers | Market Status |
|---|---|---|
| Renagel Tablets | Brand: Genzyme Generics: Various |
Available |
| Sevelamer Powder | Brand: Genzyme Generics: Various |
Available |
Consultation with healthcare professionals can provide guidance on specific product choices and experiences in different locations. Staying informed about pricing and availability can lead to better health management outcomes. Regularly checking pharmacy inventories ensures that patients have access to their required medications continuously.
Potential Manufacturers of Renagel Generics
Several pharmaceutical companies are expected to produce generics of Renagel (sevelamer hydrochloride), which is used primarily for managing phosphate levels in patients with chronic kidney disease. These manufacturers have robust track records in developing high-quality generics.
Among the potential competitors, Mylan and Teva Pharmaceuticals stand out. Both companies have a history of launching successful generics and are known for their commitment to quality and affordability. They typically conduct thorough research and adhere to rigorous FDA guidelines, ensuring that their products meet the required standards.
Another promising company is Amgen, which may look to enter this market due to its established presence in renal care. Their expertise in biologics could lend itself well to developing effective generic alternatives.
Sun Pharmaceutical Industries might also be a strong contender, given its extensive portfolio and focus on renal therapies. Their experience in manufacturing and distribution can facilitate timely access to Renagel generics across various markets.
Additionally, Lupin Pharmaceuticals has been proactive in expanding its generics division, making it a likely candidate for entering the Renagel generic space. Their competitive pricing strategies may appeal to cost-sensitive patients and healthcare systems.
Future market dynamics will depend on patent expirations and regulatory approvals, but the presence of these reputable manufacturers indicates a promising avenue for affordable Renagel alternatives. Monitoring their developments can provide insights into availability and pricing for consumers and healthcare professionals alike.
Regulatory Processes for Generic Approval of Renagel
The approval of a generic version of Renagel involves several key regulatory steps. Manufacturers must submit an Abbreviated New Drug Application (ANDA) to the FDA, demonstrating that their product is bioequivalent to the brand-name version. This includes providing data on the formulation, manufacturing processes, and labeling.
Submission and Review
Initial submission requires comprehensive documentation. Companies must present evidence from pharmacokinetic studies showing that their generic formulation delivers the same therapeutic effects as Renagel. This includes a rigorous analysis of active ingredients and excipients to ensure safety and efficacy.
Once submitted, the FDA conducts a thorough review. The review team assesses compliance with current Good Manufacturing Practices (cGMP) and other regulatory guidelines. This stage may involve inspections of manufacturing facilities to confirm adherence to safety standards.
Market Entry and Post-Market Surveillance
Upon receiving approval, manufacturers can enter the market. However, post-market surveillance remains crucial. Companies must monitor their products and report any adverse events to the FDA. This assists in maintaining safety and effectiveness in the long term.
Understanding the regulatory landscape is essential for manufacturers aiming to successfully launch a generic version of Renagel, ensuring they meet all necessary requirements for approval and market entry.
Implications of Generic Availability on Pricing
The introduction of a generic version of Renagel can lead to significant price reductions in the market. Generic drugs typically enter the market at lower prices due to reduced research and development costs and increased competition. This shift urges brand-name manufacturers to reevaluate their pricing strategies to remain competitive.
Consumers benefit directly as pharmacies adjust their pricing, often displaying the generic option alongside the brand-name product. This visibility allows patients to make more informed choices that can lead to substantial savings. Insurers may also leverage generic availability, potentially adjusting co-pays to incentivize the use of lower-cost alternatives.
Pharmaceutical companies may respond to generic competition by enhancing patient assistance programs, ensuring access while maintaining a focus on brand loyalty. Additionally, marketing campaigns targeting healthcare providers can shift to highlight the benefits and availability of generics, aiming to influence prescribing habits.
Retailers play a critical role in the pricing of generics. They may negotiate with suppliers to secure better pricing, allowing for promotions and discounts that further drive down costs for consumers. Monitoring these trends is essential for understanding the overall impact on drug affordability and access.
In summary, the availability of a generic Renagel not only reduces costs for consumers and insurers but also compels brand-name drugs to adapt. This dynamic creates a more competitive environment that ultimately benefits everyone involved in the healthcare system.
Comparative Efficacy of Renagel and Its Generics
Renagel, known for its role in managing phosphate levels in patients with kidney disease, shows comparable efficacy to its generics. Studies indicate that the active ingredient, sevelamer, remains consistent across different brands, allowing generics to offer similar outcomes in terms of phosphate control.
Clinical Evidence
Research demonstrates that generics of Renagel deliver equivalent phosphate-binding effects. In clinical trials, both Renagel and its generics achieved similar reductions in serum phosphate levels. This equivalence is crucial for healthcare providers and patients seeking effective treatment options.
Patient Experience
- Adherence: Patients often report similar experiences with both branded and generic forms, indicating no significant differences in side effects.
- Dosing: The dosing regimens for Renagel and its generics align, allowing for seamless transitions between products without compromising efficacy.
- Cost-effectiveness: Generics typically offer a more affordable option, enhancing accessibility for patients without sacrificing treatment outcomes.
Health professionals encourage monitoring serum phosphate levels consistently, regardless of the brand. Regular follow-ups ensure that patients maintain optimal control over their condition while maximizing the benefits of their prescribed therapy.